MACHINERY
 MACHINERY
MACHINERY
 MACHINERY FOR BOTTLES & CONTAINERS
MACHINERY FOR BOTTLES & CONTAINERS
 ASEPTIC B.F.S PACKAGING MACHINE
ASEPTIC B.F.S PACKAGING MACHINE
ASEPTIC B.F.S PACKAGING MACHINE
MAIN CONCEPT OF THE PLAMA PMEMS-BFS SERIES MACHINE.
PLAMA-FULLY AUTOMATIC ASEPTIC BFS MACHINE MODEL PMEMS-BFS-80D3TM WITH TRIPLE(3) PARISON DIE HEAD ON DOUBLE STATION WITH PARALLEL MOVEMENT MOULD PLATEN.
PLAMA FULLY AUTOMATIC ASEPTIC BFS MACHINE SYSTEMS (PMEMS-BFS SERIES) ARE CONSISTED OF THREE STEPS AS LIKE BLOWING THE BOTTLES(BOTTLE MAKING), LIQUID FILLING AND NECK SEALING IN ONE OPERATING CYCLE. THIS BLOWING, FILLING AND SEALING ONE TIME DOSE MEDICINE APPLICATIONS HAVE SOME ADVANTAGES AGAINST TRADITIONAL ASEPTIC FILLING IN GLASS AMPOULE. THE BFS MANUFACTURING PROCESS REFERS TO THE MAKING AND PACKAGING OF STERILE LIQUIDS WHEREIN THE BLOWING OF THE BOTTLES, THE BOTTLES FILLING WITH LIQUID, AND FORMATION OF THE SEAL TO THE BOTTLES ARE ACHIEVED ASEPTICALLY AND IN AN UNINTERRUPTED SEQUENCE OF OPERATIONS WITHOUT EXPOSING THE BOTTLES OR BOTTLES CONTENTS TO NON STERILE ENVIRONMENTS. MOST OF THE THERMOPLASTIC MATERIALS LIKE LDPE, HDPE AND PP RESIN CAN BE EASILY PROCESS.
PLAMA PMEMS-BFS MACHINE SERIES CAN BE PRODUCE FROM 0.5 ml TO 30 ml ASEPTIC BFS AMPULES. OUTPUT CAPACITY OF EACH KINDS OF SVA PRODUCTS ARE VERY DIVERSE ACCORDING TO THE NUMBER OF THE MULTI- BLOCK MOULDS, MOULD CAVITY, AMPULE VOLUME, AMPULE SHAPES AND NUMBER OF THE PARISON. THIS SVP ASEPTIC BFS MACHINE CAN PRODUCE FROM 4,000 AMPULES TO 4,200 AMPULES WITH 5 CAVITIES MULTI BLOCK MOULDS PER HOUR. BUT IF THE BUYER WANT TO PRODUCE MORE HIGH CAPACITY PLASTIC AMPULES, WE CAN SUPPLY ANOTHER TYPE OF ASEPTIC BFS MACHINE AND MULTI-BLOCK MOULD SYSTEM AS LIKE MULTI-HEAD MACHINE AND MULTI-CAVITIES BLOCK MOULDS.
AND THIS ASEPTIC BFS MACHINE IS ABLE TO MANUFACTURE A WIDE RANGE OF PHARMACEUTICAL, BIO-TECH, AND MEDICAL DEVICE PRODUCT TYPES, AND DAIRY PRODUCTS INCLUDING UNIT-DOSE BOTTLES
- GLUCOSE SOLUTION/-SALINE SOLUTION
- DISTILLED WATER FOR INJECTION(STERILIZED WATER VIALS)
- INJECTABLE AQUEOUS DRUG SOLUTIONS/-IRRIGATION SOLUTIONS
AND THIS BFS MACHINE SYSTEMS ARE INSTALLED TIME PRESSURE FILLING SYSTEMS FOR ACHIEVEMENT MINIMIZE THE VARIATION OF FILLING QUANTITY. AND SOME OF FILLING SYSTEM INCLUDING PUMP MAY CHANGE ACCORING TO THE FILLING LIQUID AND SOLUTIONS.
THE MOST IMPORTANT MERIT AND DE-MERIT OF THE PLASTIC VIALS AND GLASS VIALS
WHEN OPEN GLASS VIALS CONTAINERS GENERATE GLASS PARTICLES.
IF THESE PARTICLES ENTER INTO THE VIALS DURING OPENING, THE PRODUCT BECOMES CONTAMINATED AND CAN POTENTIALLY POSES EXTREME SAFETY ISSUES FOR THE USER.
BUT PLASTIC BFS AMPULES DO NOT CREATE PARTICLES WHEN OPENED AND THEREFORE ENHANCE PRODUCT SAFETY. MORE OVER, THE OPENING OF GLASS AMPULES ALSO PRESENTS POTENTIAL INJURY AND INFECTION TO HEALTH PROFESSIONALS AND PATIENTS DUE TO THE CREATION OF SHARP GLASS EDGES THAT CAN BE PRODUCED WHEN AMPULES ARE OPENED. WITH THE BFS TECHNOLOGY, INJECTABLE DRUG SOLUTIONS CAN BE PACKAGED IN BOTTLES THAT DO NOT INCORPORATE RUBBER STOPPERS INTO THE BOTTLES. STUDIES HAVE SHOWN THAT THE STOPPERS USED IN CONJUNCTION WITH THE PACKAGING OF PARENTERAL DRUGS IN GLASS BOTTLES ARE THE SOURCE OF PRODUCT RUBBER PARTICULATE CONTAMINATION, (WHEN THE STOPPERS IS PIERCED BY A NEEDLE DURING THE TRANSFER OF THE DRUG PRODUCT FROM THE GLASS VIAL TO A SYRINGE. BUT THIS ASEPTIC BFS
PLASTIC VIALS CAN BE AVOIDED RUBBER PARTICULATE CONTAMINATION AND GENERATE GLASS PARTICLES DURING OPENING THE VIALS.
PLAMA PMEMS-BFS MACHINES HAS ANOTHER VARIOUS MODELS AS UNDER
PLAMA PMEMS-BFS-80D3HTM; FROM 3-5 ml SMALL VOLUME DISTILLED WATER VIALS
- TRIPLE PARISON DIE HEAD WITH SINGLE STATION PARALLEL MOULD MOVEMENT SYSTEM
- OUTPUT CAPACITY; 4,000-4,200 AMPULES/HR
(OUTPUT CAPACITY MAY CHANGE ACCORDING TO MOULD CAPACITY AND AMPULE DESIGN.)
THIS ASEPTIC BFS MACHINE IS NEED MULTI-BLOCK MOULDING SYSTEM FOR MASS PRODUCTION OF SMALL PLASTIC VIALS. THIS ASEPTIC BFS MACHINE INSTALLED 5 CAVITIES MULTI-BLOCK MOULDS AT EACH 6 BLOCKS MOULD.
UNDER THE BFS MACHINES CAN SUPPLY BY THE BUYER REQUIREMENT
WE CAN SUPPLY BFS PACKAGING SYSTEM TOGETHER WITH MULTI-BLOCK MOULD ACCORDING TO THE BUYER REQUEST AS UNDER
- 2 HEAD SINGLE STATION WITH 2 MULTI-BLOCK MOULD(TOTAL CAVITY; 10 BOTTLES)
- 2 HEAD DOUBLE STATION WITH 4 MULTI-BLOCK MOULD(TOTAL CAVITY; 20 BOTTLES)
- 3 HEAD SINGLE STATION WITH 3 MULTI-BLOCK MOULDS(TOTAL CAVITY ; 15 BOTTLES)
- 4 HEAD SINGLE STATION WITH 4 MULTI-BLOCK MOULDS(TOTAL CAVITY ; 20 BOTTLES)
ESTIMATED CYCLE TIME; 22 seconds ~ 26 seconds
1. MAIN PARTS OF ASEPTIC B.F.S PACKAGING MACHINE

PP ASEPTIC MC1

PP ASEPTIC MC2

PP ASEPTIC MC3
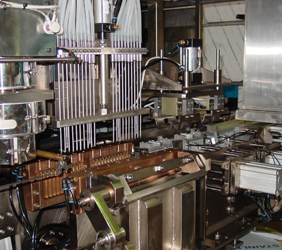
PP ASEPTIC MC4
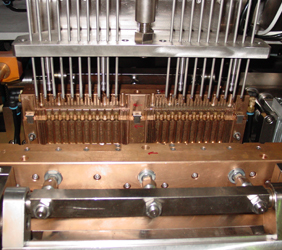
PP ASEPTIC MC5
2. VARIOUS TYPES ASEPTIC B.F.S (SMALL VOLUME)PRODUCTS

PP SVP1
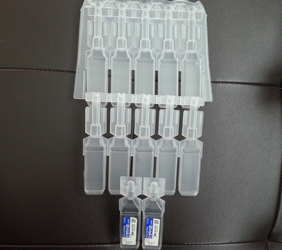
PP SVP2

PP SVP3

PP SVP4

PP SVP5
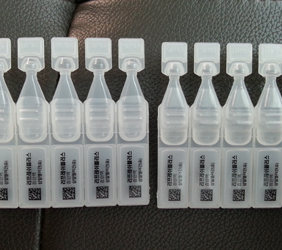
PE EYE DROPS1

PE EYE DROPS2
3. VIDEO CLIP FOR 20 ml PP ASEPTIC BOTTLES MFG. MACHINE
-
ASEPTIC PP BLOWING
4. VIDEO CLIP FOR 20 ml PP ASEPTIC FILLING-NECK SEALING-LABELLING UNIT
-
ASEPTIC PP BOTTLING
5. VIDEO CLIP FOR 0.8 ml PE EYE DROPS
-
ASEPTIC PE EYE DROPS
